Kirjeldus
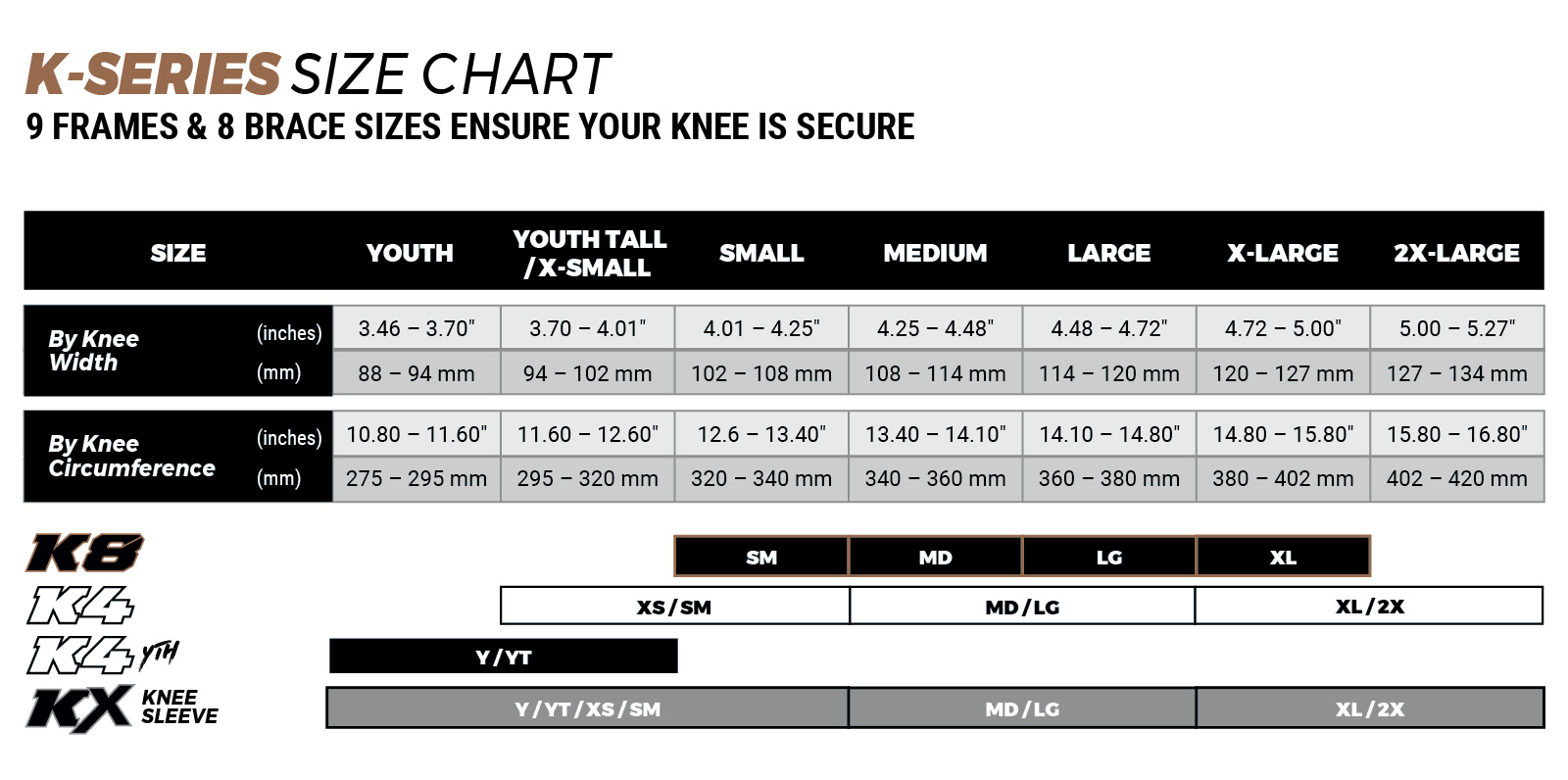
K8 2.0 KNEE BRACE
The All New K8 Knee Brace is Setting New Standards in knee protection, bringing a whole new Impact Guard design that provides better protection to your knee, a slimmer Hinge Housing with improved Ligaments, new Dampening Inserts and Knee Grippers, also featuring the new QuickLoc Clip System which allows you to anchor the brace to your leg quicker and easier.
MEDICAL COMPLIANCE
The knee is one of the largest and most complex joints in the body, with three large bones held together by four ligaments that are vital for joint stability and proper function.
Because that reason, the K8 Knee Brace Unbreakable has been certified as a medical device to help prevent leg and knee injuries such as ACL, MCL, PCL, LCL, Rotary and Combined instabilities.

POD K-Series Knee Braces are registered Medical Devices with the FDA and CE, eligibility for rebates vary based on country and region. Please contact your insurer or your local POD distributor for further information on possible reimbursement.
CODING
POD Active provides possible coding suggestions based on publicly-available information as a convenience to our customers. POD Active products that have been assigned HCPCS codes by Medicare through the coding verification process are posted below. The assigned codes are the required billing codes for these particular products. POD Active accepts no responsibility whatsoever in this regard, nor does POD Active make claims, promises or guarantees as to the availability of reimbursement for any POD Active product.
SADMERC/PDAC Letters
EU/UK (Medical Device CE/MHRA)
POD K Series – CE Declaration of Conformity (EU MDR 2017 745)
POD K Series – CE Declaration of Conformity – for the UK (MDD 93/42/EEC)
POD K Series – CE Declaration of Conformity – for the EC (MDD 93/42/EEC)
POD K Series (K1, K300, K700) – CE Declaration of Conformity